CGM recall
FreeStyle Libre 3 Constant Glucose Monitor (“CGM”) Recall Lawsuit
Understanding the Glucose Monitor Recall
The recall of a common constant glucose monitor (“CGM”) has created serious concern among people who rely on continuous glucose monitoring for safe diabetes care. The recall involves certain FreeStyle Libre Three and FreeStyle Libre Three Plus sensors that may give incorrect low readings. According to the United States Food and Drug Administration, incorrect data over time can cause people to make unsafe choices about carbohydrate intake or insulin use. The Glucose monitor recall is linked to injuries and multiple deaths reported by national news sources, including the Associated Press.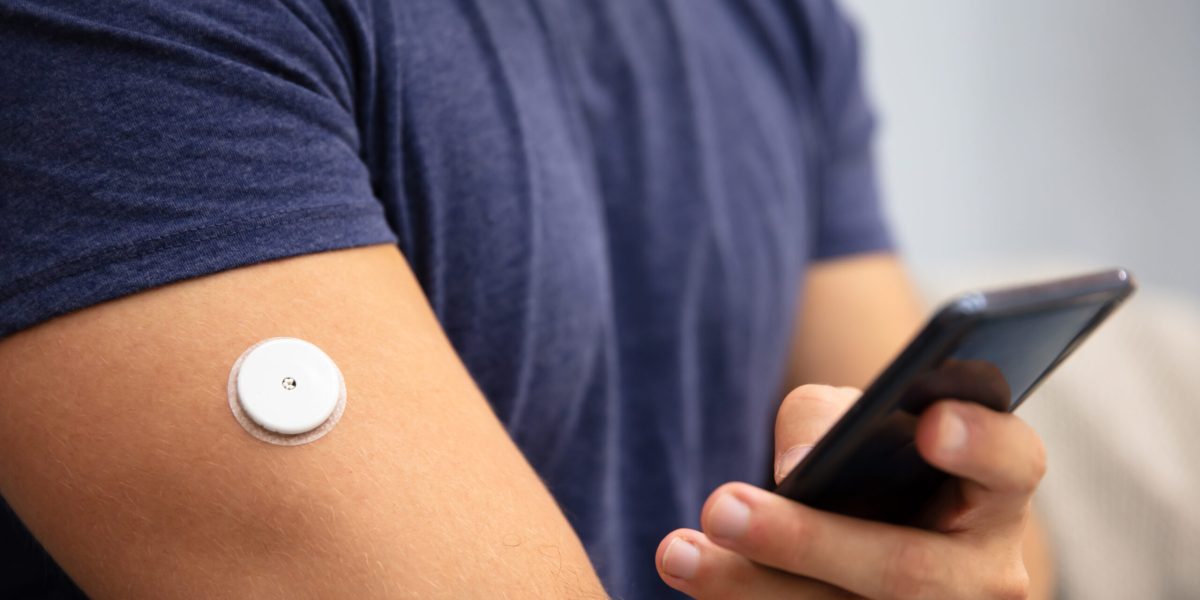
Why Trust Our Law Firm
You deserve a team that understands the science behind continuous glucose monitoring and the complex legal issues involved in a product liability lawsuit.
Our firm handles federal product liability cases across the country. Our attorneys are recognized leaders in national product injury work. Cameron Bell serves as the President of the Consumer Law Section for the Michigan State Bar which reflects our commitment to consumer safety and public responsibility.
We stand ready to help anyone affected by the Glucose monitor recall. We want to support every injured person who trusted their sensor to provide safe information.
**Note from Oliver Bell Group’s diabetic attorney**
As a Type-I diabetic, I rely on my CGM well… constantly. That’s kind of the point. I know how a diabetic’s CGM system forms a cornerstone of their life. We need to know how much insulin to take, whether we need to eat our medical candy (or if we’re lucky, cake), and just generally how we are doing during the course of the day. We trust our CGM systems with our lives and unexpected highs (because our CGM incorrectly said were low) can create real issues anywhere from just being grumpy and snapping at a colleague up to, and including, death. Oliver Bell Group is a leader in product liability litigation to begin with; in this case we also have the personal knowledge of what CGM systems mean to people beyond just numbers.
Health Risks from Faulty Glucose Monitor Sensors
Continuous glucose monitoring or CGM technology is a major part of safe diabetes management. The Cleveland Clinic explains that a CGM measures glucose in real time through the fluid under the skin. It gives twenty-four-hour data that many people depend on to prevent dangerous highs and lows.
When the sensor issues incorrect numbers serious risks can occur including severe hypoglycemia, diabetic ketoacidosis, permanent brain injury, loss of consciousness, and even death. These events are central concerns in the Glucose monitor recall.
Which Sensors Are Included in the Glucose Monitor Recall
The United States Food and Drug Administration states that Abbott Diabetes Care issued a communication to distributors, medical offices, and affected consumers about the following items:
FreeStyle Libre Three Sensor
- Model Number 72081 01
- Model Number 72080 01
- Unique Device Identifier 00357599818005
- Unique Device Identifier 00357599819002
FreeStyle Libre Three Plus Sensor
- Model Number 78768 01
- Model Number 78769 01
- Unique Device Identifier 00357599844011
- Unique Device Identifier 00357599843014
Check your sensor information here and reach out to see if you qualify for the Glucose monitor recall. We can help you confirm your device’s information.
Injured by the Glucose Monitor Recall
If you or a loved one suffered harm because of incorrect glucose data, you may qualify for a lawsuit. Injuries include dangerous low readings that trigger incorrect treatment choices and serious medical emergencies
Our firm can review your case at no cost. Product liability claims can help you recover medical expenses, lost wages, and compensation for pain and suffering.
Contact us today so we can begin helping you understand your rights in the wake of the Glucose monitor recall.
Frequently Asked Questions
- What is the Glucose Monitor Recall
The Glucose monitor recall involves specific FreeStyle Libre sensors that may report incorrect low
numbers. These incorrect readings can cause unsafe medical decisions. - Why are incorrect readings dangerous
Incorrect readings affect insulin use and carbohydrate intake. This can create severe hypoglycemia
or severe hyperglycemia which may lead to brain injury or death. - Which sensors are part of the Glucose Monitor Recall
The recall includes certain FreeStyle Libre Three and FreeStyle Libre Three Plus sensors listed by
the United States Food and Drug Administration. - How do I check if my sensor is part of the recall
Device model numbers and unique device identifiers can be found on the packaging or in your
online device account. Our team can help you verify your information. - Can I file a lawsuit because of the Glucose Monitor Recall
If you suffered injury or required medical care because of incorrect readings you may qualify for a
lawsuit. Our firm handles these cases nationwide. - What compensation can I receive
You may be able to recover medical expenses, long-term treatment costs, lost income, and
additional compensation for pain and suffering. - How much does it cost to hire your firm
There is no upfront cost. We only receive a fee when we win compensation for you.
