Cartiva Toe Implant Lawsuit
Painful Failures and Revision Surgeries
Stryker Recall
If you or a loved one suffered chronic pain, implant failure, or required revision surgery after receiving a Cartiva Synthetic Cartilage Toe Implant, you may be entitled to financial compensation. The Oliver Bell Group is actively investigating Cartiva Toe Implant Lawsuit claims nationwide.
Our firm focuses on defective medical device litigation. We are committed to holding manufacturers accountable for unsafe products placed in the market.
What Is the Cartiva Toe Implant Lawsuit?
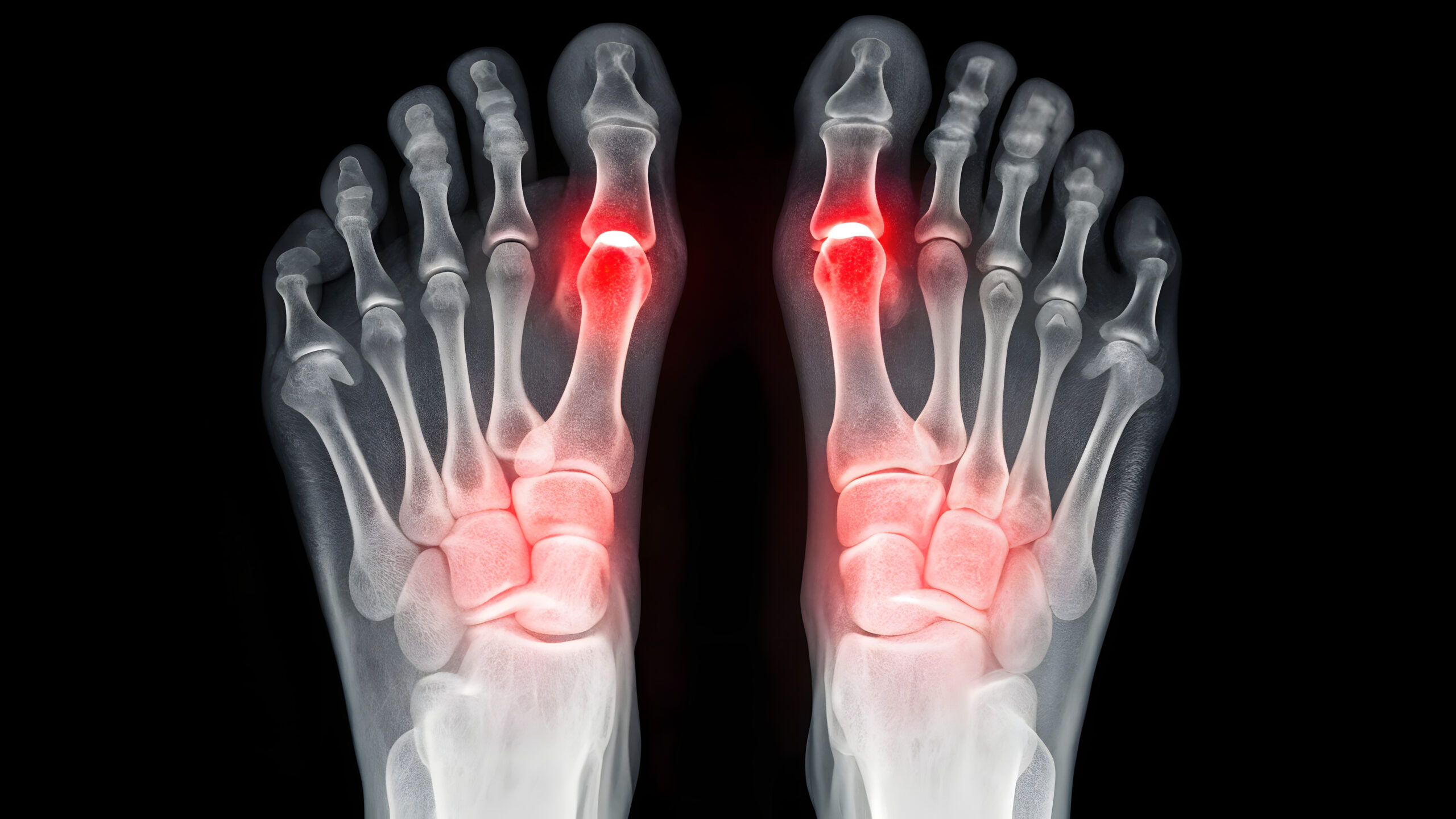
The Cartiva Toe Implant Lawsuit involves claims that the Cartiva Synthetic Cartilage Implant (SCI) was defectively designed and manufactured. This device was intended to treat arthritis of the big toe joint. However, many patients experienced serious complications and early implant failure.
Originally, Cartiva, Inc. designed and sold the implant. Later, ownership changed hands.
- Cartiva, Inc. was acquired by Wright Medical in October 2018.
- Wright Medical was acquired by Stryker Corporation in November 2020.
As a result, Stryker is legally responsible for liabilities related to Cartiva and Wright Medical implants.
The implant was sold in the United States from July 2016 through October 2024. It was later recalled.
FDA Recall Details – Cartiva Synthetic Cartilage Implant
The FDA issued a Class II recall for the Cartiva Synthetic Cartilage Toe Implant. The recall covers all manufacturing lots distributed over an eight-year period.
Recall Scope
- Distribution Dates: July 2016 to October 2024
- Affected Sizes: 6 mm, 8 mm, 10 mm, and 12 mm
- All manufacturing lots included
Affected Device Identifiers
- PDI 00852897002328 – CAR-06-US (6mm)
- PDI 00852897002021 – CAR-08-US (8mm)
- PDI 00852897002038 – CAR-10-US (10mm)
- PDI 00852897002335 – CAR-12-US (12mm)
What This Means for Patients
Although the implant was marketed as a motion-preserving alternative to toe fusion, many patients report different outcomes. In fact, physicians and patients have documented failure rates far higher than those disclosed in clinical trials.
Additionally, the polyvinyl alcohol hydrogel material, designed to mimic natural cartilage, has shown significant problems. These problems include subsidence, bone loss, and chronic pain. As a result, many patients did not achieve long-term joint preservation.
Side Effects from Recalled Cartiva SCI
According to FDA device approval documentation, reported complications include:
- Fractures
- Implant loosening, dislodgement, or displacement
- Incorrect placement or incomplete correction
- Joint instability or malalignment
- Chronic pain
- Swelling and effusion
- Bone loss and cyst formation
- Infection and blood loss
- Scarring
- Damage to nerves, arteries, or veins
- Toe numbness
- Recurrence of deformity
- Implant subsidence
- Reduced mobility and stiffness
Injuries Associated with the Cartiva Toe Implant
Patients across the United States have reported serious and life-altering injuries. These injuries include:
- Complete implant failure
- Revision surgery or toe fusion (arthrodesis)
- Implant subsidence into bone
- Bone lysis around the implant site
- Chronic pain and swelling
- Nerve damage or fragmentation
- Loss of mobility and stiffness
- Inability to walk without assistance
Clinical Evidence of High Failure Rates
A peer-reviewed study published in Cureus evaluated surgical outcomes for Cartiva implants. The findings were concerning.
45.5% total complication rate
18.2% reoperation rate
55% of patients reported continued pain
50% experienced stiffness or reduced motion
15% reported neurovascular symptoms
Qualifying Injuries and Steps to Take
If you received a Cartiva toe implant and experienced complications, take action as soon as possible.
Steps to Follow
Document symptoms. Record pain, swelling, stiffness, or nerve issues.
Collect medical records. Include surgical notes, imaging, and revision procedures.
Do not delay. Statutes of limitation apply.
Contact a Cartiva Toe Implant lawyer.
Type of Product Liability Case
The Cartiva Toe Implant Lawsuit is a product liability and defective medical device case. Claims may include:
- Defective design
- Failure to warn
- Misrepresentation of safety and longevity
- Corporate successor liability against Stryker
Why Choose the Oliver Bell Group?
The Oliver Bell Group has extensive experience representing victims of defective medical devices and recalled implants.
- Our Commitment
- In-depth case investigation
- Medical expert review
- Aggressive manufacturer accountability
- No fees unless we win
- We pursue compensation for:
- Medical expenses
- Revision surgeries
- Lost wages
- Pain and suffering
- Permanent disability
Contact Us Today – Free Case Review
If you or a loved one were harmed by a Cartiva Synthetic Cartilage Toe Implant, contact the Oliver Bell Group today. We offer a free and confidential consultation. We are actively reviewing Cartiva Toe Implant Lawsuit claims nationwide.
FAQs
What is the Cartiva Toe Implant Lawsuit about?
The lawsuit involves claims that the Cartiva Synthetic Cartilage Implant was defectively designed and caused serious orthopedic complications.
Who is responsible for the Cartiva implant?
Stryker Corporation is responsible after acquiring Wright Medical, which previously acquired Cartiva, Inc.
What injuries qualify for a lawsuit?
Qualifying injuries include implant failure, chronic pain, bone loss, nerve damage, revision surgery, or toe fusion.
What type of lawsuit is this?
This is a defective medical device and product liability lawsuit.
How do I start a Cartiva Toe Implant claim?
Contact the Oliver Bell Group for a free consultation. Our attorneys will review your case and explain your legal options.
